Connected worker is essential to realizing the full impact of digital transformation
The connected worker is essential to realizing the full impact of digital transformation
LNS Research’s ebook “The Connected Worker: Connecting People and Systems to Transform Frontline Operations” outlines their reasoning why connected worker initiatives are not only strategic in 2020 but also an essential part of realizing digital transformation in the next decade.
But first, let’s define what is meant by a “connected worker”. First off who are these workers? Are they a selected few, responsible for the most intricate of business functions? No, the connected worker is anyone involved in front-line operations for a business. That means the connected worker is someone in a manufacturing plant, in the field, or at a customer’s location. The connected worker is anyone involved in the production of goods or delivery of services. Given this, initiatives that connect the everyday worker will have overreaching and significant business impact.
What does connected mean? The ‘connection’ being referenced is an availability of business data being made by new technology solutions that will get implemented in the workplace. This ‘connected data’ and information comes in the form of digital threads, providing answers to some basic question of who, what and why for the business. Examples of threads include information related to digital work instructions, machinery metrics, and automated risk, safety, audit and training information.
The scope of connected worker initiatives, the use cases being addressed and the benefits that connected worker solutions provide is broad. LNS Research outlines them into the following:
LNS Research: Connected Worker: Operations-Wide Scope and Benefits
Connected worker solutions improve not only the bottom-line operations of the organization, but also provide intrinsic value to the worker himself. Connected worker solutions fundamentally change what the worker does on a daily basis. Previous mundane, rote and uninformed routine behaviors are now traded in for high value, data driven activities with real-time data visibility and decision making. As result, the worker is empowered to make better, faster decisions and be more proactive in identifying and acting on improvement opportunities.
Many companies struggle to identify which use case to focus and target for implementation. The use cases that have the highest potential help address the critical workforce demographic and skill gap challenges faced in industrial sectors. As a result, a large focus for future solutions will be mobile device applications for digital field workers and data capture in remote operation centers.
Learn more about Tego’s mobile device application for field service organizations click here.


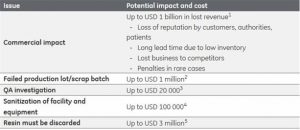

 What exactly is the 10/90 rule as it applies to the Internet of Things (IoT)? According to Dr. Mazlan Abbas at the
What exactly is the 10/90 rule as it applies to the Internet of Things (IoT)? According to Dr. Mazlan Abbas at the