“Lack of sterility assurance” in CGMP strikes again: Hospira issues a recall
We learned late last week that Pfizer subsidiary Hospira issued a nationwide recall of its vials used to inject sodium bicarbonate, citing sterility concerns. Not only will this cause undue financial damage to the company, but more critically it removes a volume of potentially lifesaving drug from the market.
There’s a downstream impact, as well. PharMEDium Services had to recall a full run of its products that had been compounded using the affected Hospira lots. One sterility monitoring mix-up upstream creates a domino effect across the entire value chain.
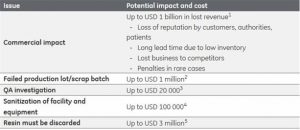
Again, the difficulty manufacturers have with proving sterility across the entirety of their processes creates an outsized burden, not only for the manufacturer itself, but also for its partners and the healthcare community at-large. Unfortunately, the Hospira recall is but a time-stamped snapshot into how bioburden incidents add up annually, outlined in early Q2 2017 by Bioprocess Online.
Most importantly, this instance is a harsh reminder that a solvable issue continues to present a significant challenge. The good news is, there are new ways to overcome it. By storing digital data directly on a facility’s manufacturing components, monitoring processes can become automated and touchless, and manufacturers are more able to account for the possibility of contamination before lots are released to the market. This goes to mitigate against the potential for costly, brand damaging situations to occur. There’s much less chance for sterility to be compromised because operator touches have been reduced and digital information has been made readily available for easy access. If something’s askew, it is much more likely to be caught before final product leaves the building.
To learn about Tego’s sterilization-proof monitoring solutions for pharmaceutical manufacturing, please visit this page.
To schedule a demo and see if Tego can improve your aseptic manufacturing processes, contact us here.