Category: Blog
-
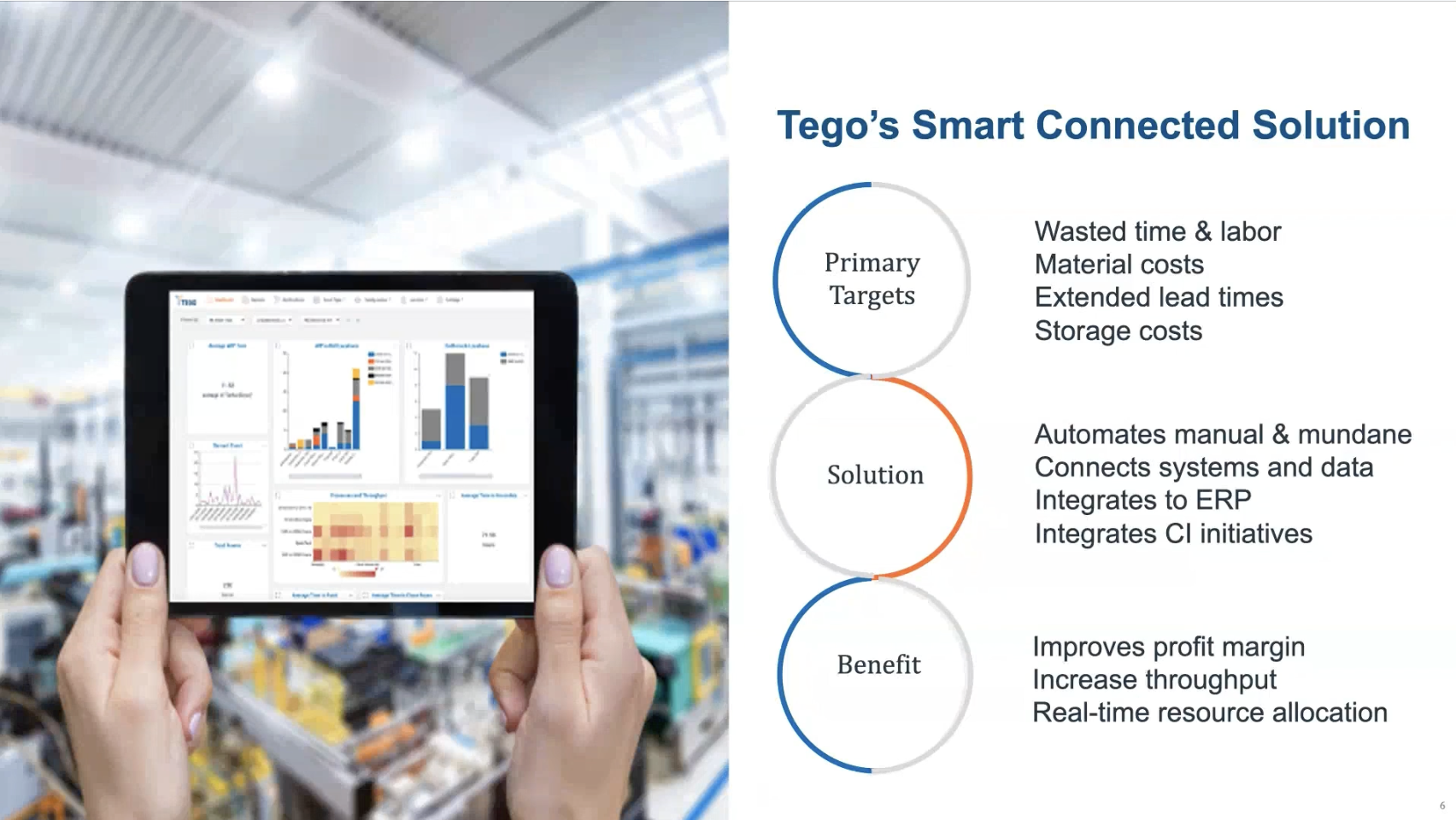
Empowering Industrial Manufacturers with Passive Real-Time Location Data For Profitability, Competitiveness and Market Growth
Industrial teams are under pressure to hit dates, reduce waste, and protect margins. The hard part isn’t collecting more data. It’s turning what you…
-

Unlock Real-Time Operational Precision with Tego Inc. + Zebra Technologies
In today’s industrial landscape, the difference between success and stagnation is visibility. Industrial leaders are under pressure to streamline operations, eliminate data silos, and…
-

Tego Inc and TSC Printronix Auto ID Partner to Offer Complete Edge to Cloud Solution
The Partnership Serves Digital Transformation Initiatives in the Aviation, Automotive, Rail, and Healthcare Industries WALTHAM MA SEPTEMBER 23, 2020 – Tego Inc announces…
