Solutions
Aseptic Manufacturing
Environmental monitoring (EM) programs that rely on manual processes experience errors, delays, and costly investigations. In an industry where quality impacts lives, real-time visibility and automated quality assurance are paramount for preventing contamination and ensuring product safety. Tego’s sterilization-proof digital solution offers automated, real-time tracking, alerting and analytics, which eliminates paper trails and enables immediate issue resolution.
Tego’s aseptic manufacturing solution uses sterilization-proof RFID, automation, and real-time analytics for contamination-free operations, full traceability, rapid issue resolution, and consistent compliance.

Core Benefits
Establish an automated, end to end, paperless environmental monitoring (EM) program for aseptic manufacturers. Support quality control with complete digital transparency and automated reporting throughout the aseptic manufacturing and product release process.
Rely on Tego for digitizing settle plates used for environmental monitoring programs, which enables real-time visibility, traceability and analytics. The solution empowers biotechnology and pharmaceutical companies to attain a fully digital environmental monitoring program.
- Reduce manual data collection, redundant data entry, and transcription errors by 50%
- Improve Quality Control (QC) worker’s efficiency by 30%
- Gain direct traceability of QC Micro programs
- Maintain a paperless regulatory compliant workspace, which increases speed to market
- Reduce investigations by 28%
- Make sound product and quality release decisions
- Reduce errors with automated data flowing from the manufacturing floor into LIMS
- Gain real-time alerting and event reporting based on movement of aseptic materials and samples
- Personnel monitoring in real time reduces contaminant exposure by 60%
PROOF POINTS
Real Results in High-Stakes Industries

Digital Transformation in a Blood Plasma Pharmaceutical Company
For global biopharmaceutical manufacturers, ensuring cradle-to-grave traceability is both a regulatory necessity and a massive operational challenge. Managing millions of plasma collection containers across…
Learn More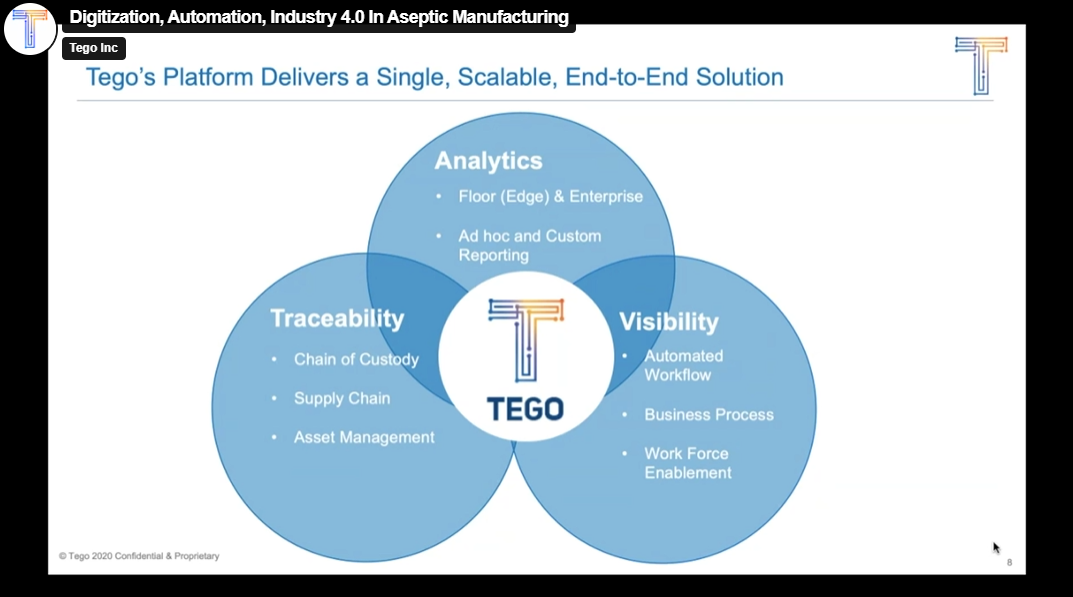
Digitization, Automation, and Industry 4.0 in Aseptic Manufacturing
Aseptic manufacturers are under more pressure than ever. Continuous manufacturing and the rise of precision medicine (like CAR-T therapies) are creating complex production cycles…
Learn MoreNews and Insights
Bring Full Traceability to Aseptic Manufacturing
Are you ready to harness the power of RFID digitalization in your aseptic manufacturing setting? Unlock the full potential with Tego’s proven solution.